Biosimilars – Pharmakokinetic studies in healthy subjects
Why Germany for PK biosimilar studies?
Germany offers excellent recruitment potential for studies with high sample sizes in healthy subjects: more than 80 million inhabitants, a very high population density and large urban centers. People are socially well embedded, the infrastructure is reliable, and high quality standards ensure low drop-out rates and high data quality.
How do we recruit in Germany?
SocraTec R&D has successfully established a network of professional phase-I CROs for PK studies: All participating phase-I CROs enjoy an excellent reputation, combine decades of experience and share a successful inspection history. They are located in different densely populated areas of Germany and all have very good volunteer databases. Excellent recruitment numbers speak for themselves. With permission of the very satisfied client we present the numbers of a successful project with a total of 491 randomized subjects:
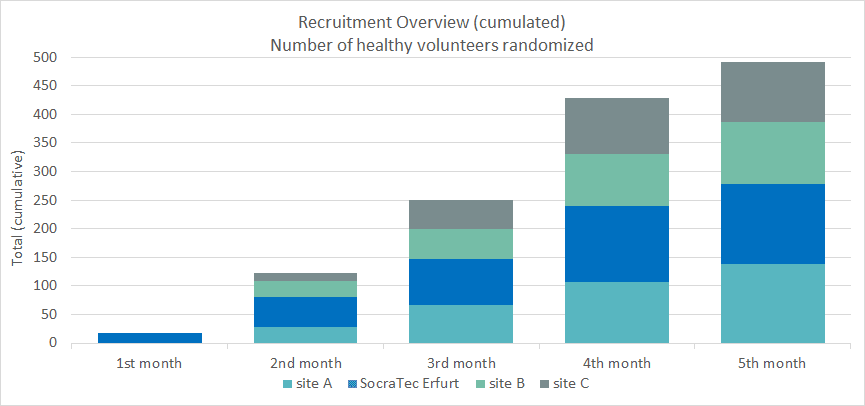
Recruitment of 491 subjects randomized within less than 5 months including 4 sites in Germany.
What does SocraTec R&D do as lead CRO?
- Trial set-up and planning including sample size estimation and protocol writing
- Trial submission with fast and efficient regulatory and ethic’s approval (26 days for mono-national studies in Germany)
- Clinical performance of a significant number of subjects in the own clinical pharmacology unit (in the example above we had the fastest recruitment rate of all CPUs)
- Coordination of all sites in a competitive recruitment
- Complete project responsibility including sponsor’s oversight
- Continuous optimization of study coordination and recruitment
- Data management with a highly efficient eCRF, PK and statistics (Incl. SDTM and ADaM for FDA submission)
Optimal conditions for bioequivalence studies with biosimilars in Germany!
Interested?
Then contact us to find out more.
